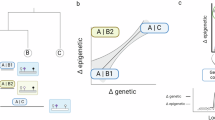
Abstract
Phenotypic variation is traditionally parsed into components that are directed by genetic and environmental variation. The line between these two components is blurred by inherited epigenetic variation, which is potentially sensitive to environmental inputs. Chromatin and DNA methylation-based mechanisms mediate a semi-independent epigenetic inheritance system at the interface between genetic control and the environment. Should the existence of inherited epigenetic variation alter our thinking about evolutionary change?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bird, A. & Macleod, D. Reading the DNA methylation signal. Cold Spring Harb. Symp. Quant. Biol. 69, 113–118 (2004).
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genet. 33, S245–S254 (2003).
Wang, Y. et al. Linking covalent histone modifications to epigenetics: the rigidity and plasticity of the marks. Cold Spring Harb. Symp. Quant. Biol. 69, 161–169 (2004).
Bernstein, E. & Allis, C. D. RNA meets chromatin. Genes Dev. 19, 1635–1655 (2005).
Matzke, M. A. & Birchler, J. A. RNAi-mediated pathways in the nucleus. Nature Rev. Genet. 6, 24–35 (2005).
Wassenegger, M. The role of the RNAi machinery in heterochromatin formation. Cell 122, 13–16 (2005).
Di Croce, L. et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science 295, 1079–1082 (2002).
Jia, S., Noma, K. & Grewal, S. I. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 304, 1971–1976 (2004).
Makar, K. W. et al. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nature Immunol. 4, 1183–1190 (2003).
Robertson, K. D. et al. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nature Genet. 25, 338–342 (2000).
Yamada, T., Fischle, W., Sugiyama, T., Allis, C. D. & Grewal, S. I. The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol. Cell 20, 173–185 (2005).
Grace Goll, M. & Bestor, T. H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 74, 481–514 (2005).
Genereux, D. P., Miner, B. E., Bergstrom, C. T. & Laird, C. D. A population-epigenetic model to infer site-specific methylation rates from double-stranded DNA methylation patterns. Proc. Natl Acad. Sci. USA 102, 5802–5807 (2005).
Pfeifer, G. P., Steigerwald, S. D., Hansen, R. S., Gartler, S. M. & Riggs, A. D. Polymerase chain reaction-aided genomic sequencing of an X chromosome-linked CpG island: methylation patterns suggest clonal inheritance, CpG site autonomy, and an explanation of activity state stability. Proc. Natl Acad. Sci. USA 87, 8252–8256 (1990).
Boyes, J. & Bird, A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 11, 327–333 (1992).
Lorincz, M. C., Schubeler, D., Hutchinson, S. R., Dickerson, D. R. & Groudine, M. DNA methylation density influences the stability of an epigenetic imprint and Dnmt3a/b-independent de novo methylation. Mol. Cell. Biol. 22, 7572–7580 (2002).
Schotta, G. et al. Central role of Drosophila SU(VAR)3–9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 21, 1121–1131 (2002).
Richards, E. J. & Elgin, S. C. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108, 489–500 (2002).
Selker, E. U. et al. Induction and maintenance of nonsymmetrical DNA methylation in Neurospora. Proc. Natl Acad. Sci. USA 99 (Suppl. 4), 16485–16490 (2002).
Sugiyama, T., Cam, H., Verdel, A., Moazed, D. & Grewal, S. I. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc. Natl Acad. Sci. USA 102, 152–157 (2005).
Kahan, B. & DeMars, R. Autonomous gene expression on the human inactive X chromosome. Somatic Cell Genet. 6, 309–323 (1980).
Morgan, H. D., Santos, F., Green, K., Dean, W. & Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14, R47–R58 (2005).
Jablonka, E. & Lamb, M. J. Epigenetic Inheritance and Evolution (Oxford Univ. Press, Oxford, 1995).
Macleod, D., Clark, V. H. & Bird, A. Absence of genome-wide changes in DNA methylation during development of the zebrafish. Nature Genet. 23, 139–140 (1999).
Lane, N. et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35, 88–93 (2003).
Silva, A. J. & White, R. Inheritance of allelic blueprints for methylation patterns. Cell 54, 145–152 (1988).
Holliday, R. The inheritance of epigenetic defects. Science 238, 163–170 (1987).
Lippman, Z. et al. Role of transposable elements in heterochromatin and epigenetic control. Nature 430, 471–476 (2004).
Liu, J., He, Y., Amasino, R. & Chen, X. siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in Arabidopsis. Genes Dev. 18, 2873–2878 (2004).
Michaud, E. J. et al. Differential expression of a new dominant agouti allele (Aiapy) is correlated with methylation state and is influenced by parental lineage. Genes Dev. 8, 1463–1472 (1994).
Morgan, H. D., Sutherland, H. G., Martin, D. I. & Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nature Genet. 23, 314–318 (1999).
Jacobsen, S. E., Sakai, H., Finnegan, E. J., Cao, X. & Meyerowitz, E. M. Ectopic hypermethylation of flower-specific genes in Arabidopsis. Curr. Biol. 10, 179–186 (2000).
Kakutani, T., Jeddeloh, J. A., Flowers, S. K., Munakata, K. & Richards, E. J. Developmental abnormalities and epimutations associated with DNA hypomethylation mutations. Proc. Natl Acad. Sci. USA 93, 12406–12411 (1996).
Kankel, M. W. et al. Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163, 1109–1122 (2003).
Fraga, M. F. et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl Acad. Sci. USA 102, 10604–10609 (2005).
Feinberg, A. P. The epigenetics of cancer etiology. Semin. Cancer Biol. 14, 427–432 (2004).
Bastow, R. et al. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427, 164–167 (2004).
Steward, N., Ito, M., Yamaguchi, Y., Koizumi, N. & Sano, H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J. Biol. Chem. 277, 37741–37746 (2002).
Axtell, J. D. & Brink, R. A. Chemically induced paramutation at the R locus in maize. Proc. Natl Acad. Sci. USA 58, 181–187 (1967).
Ivarie, R. D. & Morris, J. A. Induction of prolactin-deficient variants of GH3 rat pituitary tumor cells by ethyl methanesulfonate: reversion by 5-azacytidine, a DNA methylation inhibitor. Proc. Natl Acad. Sci. USA 79, 2967–2670 (1982).
Stokes, T. L., Kunkel, B. N. & Richards, E. J. Epigenetic variation in Arabidopsis disease resistance. Genes Dev. 16, 171–182 (2002).
Jacobsen, S. E. & Meyerowitz, E. M. Hypermethylated SUPERMAN epigenetic alleles in Arabidopsis. Science 277, 1100–1103 (1997).
Soppe, W. J. et al. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol. Cell 6, 791–802 (2000).
Cooney, C. A., Dave, A. A. & Wolff, G. L. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 132, 2393S–2400S (2002).
Waterland, R. A. & Jirtle, R. L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 23, 5293–5300 (2003).
Wolff, G. L., Kodell, R. L., Moore, S. R. & Cooney, C. A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 12, 949–957 (1998).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nature Neurosci. 7, 847–854 (2004).
Anway, M. D., Cupp, A. S., Uzumcu, M. & Skinner, M. K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005).
Roemer, I., Reik, W., Dean, W. & Klose, J. Epigenetic inheritance in the mouse. Curr. Biol. 7, 277–280 (1997).
Carrel, L. & Willard, H. F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 (2005).
Cubas, P., Vincent, C. & Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401, 157–161 (1999).
Waddington, C. H. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (1942).
Waddington, C. H. Genetic assimilation of an acquired character. Evolution 7, 118–126 (1953).
Pal, C. & Miklos, I. Epigenetic inheritance, genetic assimilation and speciation. J. Theor. Biol. 200, 19–37 (1999).
Provine, W. B. The Origins of Theoretical Population Genetics (Univ. Chicago Press, Chicago, 1971).
Denver, D. R. et al. The transcriptional consequences of mutation and natural selection in Caenorhabditis elegans. Nature Genet. 37, 544–548 (2005).
Schultz, S. T., Lynch, M. & Willis, J. H. Spontaneous deleterious mutation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 96, 11393–11398 (1999).
Shaw, R. G., Byers, D. L. & Darmo, E. Spontaneous mutational effects on reproductive traits of Arabidopsis thaliana. Genetics 155, 369–378 (2000).
Sollars, V. et al. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nature Genet. 33, 70–74 (2003).
Fazzari, M. J. & Greally, J. M. Epigenomics: beyond CpG islands. Nature Rev. Genet. 5, 446–455 (2004).
Waddington, C. H. The epigenotype. Endeavour 1, 18–20 (1942).
Filipowicz, W., Jaskiewicz, L., Kolb, F. A. & Pillai, R. S. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr. Opin. Struct. Biol. 15, 331–341 (2005).
Tijsterman, M., Ketting, R. F. & Plasterk, R. H. The genetics of RNA silencing. Annu. Rev. Genet. 36, 489–519 (2002).
Mayr, E. in The Evolutionary Synthesis (eds Mayr, E. & Provine, W. B.) 1–48 (Harvard Univ. Press, Cambridge, Massachusetts; London, England, 1980).
Mayr, E. The Growth of Biological Thought (Harvard Univ. Press, Cambridge, Massachusetts, 1982).
Burkhardt, R. W. The Spirit of System: Lamarck and Evolutionary Biology (Harvard Univ. Press, Cambridge, Massachusetts, 1995).
Banks, J. A., Masson, P. & Fedoroff, N. Molecular mechanisms in the developmental regulation of the maize Suppressor-mutator transposable element. Genes Dev. 2, 1364–1380 (1988).
Rakyan, V. K. et al. Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. Proc. Natl Acad. Sci. USA 100, 2538–2543 (2003).
Stam, M., Belele, C., Dorweiler, J. E. & Chandler, V. L. Differential chromatin structure within a tandem array 100 kb upstream of the maize b1 locus is associated with paramutation. Genes Dev. 16, 1906–1918 (2002).
Colot, V., Maloisel, L. & Rossignol, J. L. Interchromosomal transfer of epigenetic states in Ascobolus: transfer of DNA methylation is mechanistically related to homologous recombination. Cell 86, 855–864 (1996).
Suter, C. M., Martin, D. I. & Ward, R. L. Germline epimutation of MLH1 in individuals with multiple cancers. Nature Genet. 36, 497–501 (2004).
Das, O. P. & Messing, J. Variegated phenotype and developmental methylation changes of a maize allele originating from epimutation. Genetics 136, 1121–1141 (1994).
Bender, J. & Fink, G. R. Epigenetic control of an endogenous gene family is revealed by a novel blue fluorescent mutant of Arabidopsis. Cell 83, 725–734 (1995).
Melquist, S. & Bender, J. Transcription from an upstream promoter controls methylation signaling from an inverted repeat of endogenous genes in Arabidopsis. Genes Dev. 17, 2036–2047 (2003).
Acknowledgements
I am grateful for the helpful comments of G. Allen and the anonymous reviewers. My laboratory's experimental work on epigenetic variation and inheritance is funded by the US National Science Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Richards, E. Inherited epigenetic variation — revisiting soft inheritance. Nat Rev Genet 7, 395–401 (2006). https://doi.org/10.1038/nrg1834
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg1834