Abstract
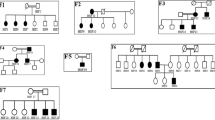
Focal segmental glomerulosclerosis (FSGS) is a pattern of kidney injury observed either as an idiopathic finding or as a consequence of underlying systemic conditions. Several genes have been identified that, when mutated, lead to inherited FSGS and/or the related nephrotic syndrome. These findings have accelerated the understanding of glomerular podocyte function and disease, motivating our search for additional FSGS genes. Using linkage analysis, we identified a locus for autosomal-dominant FSGS susceptibility on a region of chromosome 14q. By sequencing multiple genes in this region, we detected nine independent nonconservative missense mutations in INF2, which encodes a member of the formin family of actin-regulating proteins. These mutations, all within the diaphanous inhibitory domain of INF2, segregate with FSGS in 11 unrelated families and alter highly conserved amino acid residues. The observation that alterations in this podocyte-expressed formin cause FSGS emphasizes the importance of fine regulation of actin polymerization in podocyte function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 February 2010
In the version of this article initially published, Stephen J. Tonna was inadvertently omitted from the author list, and the fourth author (Hiroyasu Tsukaguchi) was missing one of his affiliations. These errors have been corrected in the HTML and PDF versions of the article.
References
Kaplan, J.M. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Winn, M.P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Chhabra, E.S. & Higgs, H.N. INF2 is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J. Biol. Chem. 281, 26754–26767 (2006).
D'Agati, V.D., Fogo, A.B., Bruijn, J.A. & Jennette, J.C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am. J. Kidney Dis. 43, 368–382 (2004).
Kent, W.J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Chhabra, E.S., Ramabhadran, V., Gerber, S.A. & Higgs, H.N. INF2 is an endoplasmic reticulum-associated formin protein. J. Cell Sci. 122, 1430–1440 (2009).
Faix, J. & Grosse, R. Staying in shape with formins. Dev. Cell 10, 693–706 (2006).
Li, F. & Higgs, H.N. The mouse formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 (2003).
Rose, R. et al. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435, 513–518 (2005).
Seth, A., Otomo, C. & Rosen, M.K. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLα and mDia1. J. Cell Biol. 174, 701–713 (2006).
Higgs, H.N. Formin proteins: a domain-based approach. Trends Biochem. Sci. 30, 342–353 (2005).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Huber, T.B. & Benzing, T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr. Opin. Nephrol. Hypertens. 14, 211–216 (2005).
Schäffer, A.A. Faster linkage analysis computations for pedigrees with loops or unused alleles. Hum. Hered. 46, 226–235 (1996).
O'Connell, J.R. & Weeks, D.E. The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat. Genet. 11, 402–408 (1995).
Markianos, K., Daly, M.J. & Kruglyak, L. Efficient multipoint linkage analysis through reduction of inheritance space. Am. J. Hum. Genet. 68, 963–977 (2001).
Kelley, L.A. & Sternberg, M.J. Protein structure prediction on the web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 (2009).
Otomo, T. et al. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 433, 488–494 (2005).
Lammers, M., Rose, R., Scrima, A. & Wittinghofer, A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J. 24, 4176–4187 (2005).
Nezami, A.G., Poy, F. & Eck, M.J. Structure of the autoinhibitory switch in formin mDia1. Structure 14, 257–263 (2006).
Berger, U.V. & Hediger, M.A. Differential distribution of the glutamate transporters GLT-1 and GLAST in tanycytes of the third ventricle. J. Comp. Neurol. 433, 101–114 (2001).
Acknowledgements
We thank the many individuals who participated in this study; S. DePalma for help with linkage analysis; and J. Jacobs, K. Tucker, J. Holt, A. Acharya, R.Wiggins, R.Weir, J. Levine and many others for help with family ascertainment. This work was support by grants from the US National Institutes of Health (DK54931 to M.R.P., DK073091 to J.M.H., DK080947 to J.S.S. and GM069818 to H.N.H.) and the Clinical Investigator Training Program: Beth Israel Deaconess Medical School in collaboration with Pfizer Inc. and Merck and Co., the NephCure Foundation, and the Cole Pasqualucci Nephrotic Syndrome and FSGS Research fund (to E.J.B.). M.R.P. is an Established Investigator of the American Heart Association.
Author information
Authors and Affiliations
Contributions
E.J.B. performed family and clinical ascertainment, performed and interpreted genetic linkage studies, performed mutational analysis and made DNA constructs. J.S.S. helped with study design and interpretation and performed INF2 expression studies. D.J.B. performed INF2 expression studies. H.T. performed genetic linkage studies. S.J.T. performed genotyping and sequencing analyses. A.L.U. helped with clinical ascertainment of the families. H.N.H. performed molecular modeling and assisted with data interpretation. J.M.H. performed histology studies. M.R.P. oversaw all aspects of study design and interpretation and wrote the manuscript with the assistance of the other authors.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Table 1 and Supplementary Note (PDF 781 kb)
Rights and permissions
About this article
Cite this article
Brown, E., Schlöndorff, J., Becker, D. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42, 72–76 (2010). https://doi.org/10.1038/ng.505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.505